Introduction
In 2019 Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2 or more commonly known as COVID-19) was identified in Wuhan, China. By December 2021, more than 270 million cases and more than five million deaths have been reported and the numbers keep increasing (1). A dire need for a vaccine was felt due to its higher transmission rate compared to other communicable diseases like Severe Acute Respiratory Syndrome (SARS), Middle East Respiratory Syndrome (MERS) and, Ebola. As a result, the scientific community is working together to develop a variety of vaccines at an unprecedented pace. This report attempts to improve the knowledge about COVID-19 vaccination among the general public.
What is vaccination?
Vaccination is a process where a human body is protected against harmful diseases before they come into contact by using the natural defense system of the human body to build resistance to specific harmful microbes. In this procedure the immune system is stimulated to create antibodies, just as it does when a real threat is identified (8). The effectiveness of this process can be improved by periodic repetition of injections. Vaccination has proven to be the most effective way to prevent infectious diseases. When a large portion of a population is vaccinated it leads to herd immunity. Herd immunity provides indirect protection to those who are immune-compromised and cannot get the vaccine. The threshold for herd immunity is estimated as 60-70% (2). Diseases such as smallpox, polio, and rubella, were successfully controlled using this concept.
Types of Vaccines
Vaccines are often classified into four main types by their antigen(s) and active element that generates immune response. Those are,
- Inactivated vaccines
- Live-attenuated vaccines
- Toxoid vaccines
- Subunit vaccines
Inactivated vaccines use the dead version of a virus or bacteria which causes a disease. Inactivated vaccines can be given even to a person with a weak immune system. Examples are Hepatitis, influenza, and polio.
Live-attenuated vaccines contain alive but weakened viruses or bacteria so they can replicate without causing the disease. If administered to an immune-suppressed person, it could lead to a severe condition due to uncontrolled replication. Examples are vaccines for Measles, smallpox, chickenpox.
Toxins made by pathogenic microbes are used in toxoid vaccines. Toxoids are modified versions of toxins which are no longer toxic. Toxoids allow the body to maintain an immunological response to the original toxin. Since the toxoid is a weaker form of the toxin, it cannot cause toxicity or disease. Examples are Tetanus and Diphtheria.
Subunit vaccines use specific parts of a virus or bacteria such as protein, polysaccharide, and nucleic acid. Since a specific part of the germ is used, very strong immunity is achieved through this. This is used to vaccinate immune-suppressed people. In the case of COVID-19, many RNA and DNA vaccines have been developed in recent times. When we consider mRNA vaccine it only carries specific information required to create a part of the pathogen, not the whole unit. This allows developing a specific immune response to a certain pathogen.
Stages of Vaccine Development
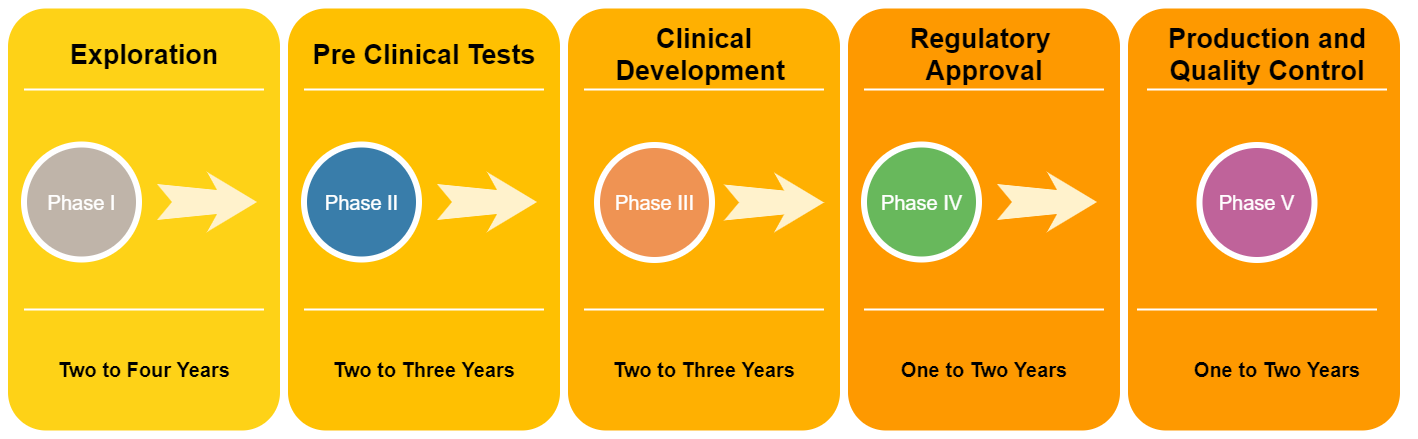
The development of vaccines is a complicated and lengthy process (Figure 01) that consists of several stages. The main five stages can be listed as:
- Exploration
- Pre-clinical Tests
- Clinical Development
- Regulatory Approval
- Production and quality control
Stage 01: Exploration
A phase where laboratory research and development is done to identify certain antigens, which are capable of triggering the immune system of a human in order to produce antibodies. This process usually takes two to four years to complete.
Stage 02: Pre-Clinical Tests
In this phase, experiments are conducted on cells, tissues, and animals. From these tests, the efficacy, effectiveness, and the immunogenicity of a certain vaccine can be identified. This process usually takes one to two years to complete.
Stage 03: Clinical Development
This step is carried out by a sponsor, mostly a private company under the supervision of an institution that will host clinical trials. Consists of three major Phases;
Phase I: A small group of people (20-80 volunteers) are injected with the vaccine to determine the safety, efficacy, side effects, and most importantly the immune response.
Phase II: The vaccine will be proceeding to the next level if the results in the previous phase are positive. A wider group of randomly selected people (more than 100) are included in this phase. Immunogenicity, safety, and placebo effect are determined.
Phase III: More than thousands of humans are administered with the vaccine. Efficacy, safety, and rare side effects are determined.
Stage 04: Regulatory Approval
This is the stage wherever the drug developer submits an application for the approval of the regulatory authority when passing all the above-mentioned phases.
Stage 05: Production and quality control
After the vaccine has been registered for use, manufacturers provide details necessary to produce a vast amount of quantities of vaccines. Quality control is the last step where even after the distribution of the vaccine, Phase IV trials are conducted in order to monitor the safety, performance, effectiveness, and efficacy of the vaccine. This is done to track and check whether a vaccine is performing as expected.
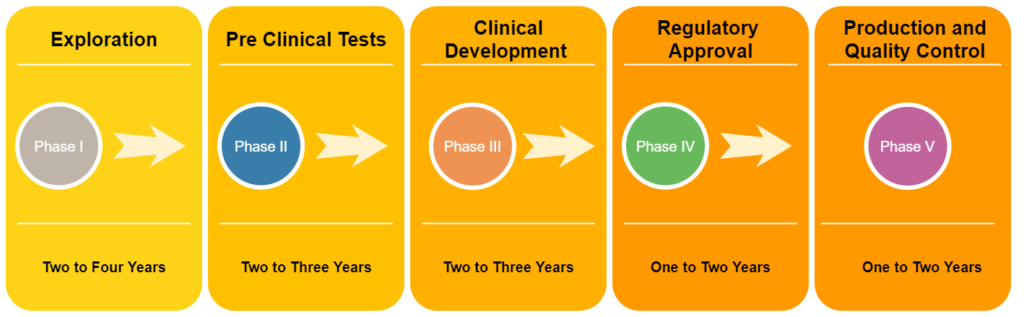
According to Figure 01, vaccine development usually takes 10-15 years on average to complete. But in the case of COVID-19, few companies have managed to develop and distribute effective vaccines in less than two years (Figure 02). The normal vaccine development process performs each step in sequential order. But in the development of COVID-19 vaccines scientists have performed steps in a parallel order to accelerate the process.
Efficacy and effectiveness
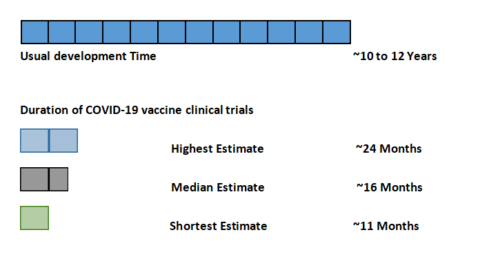
Efficacy and effectiveness are parameters that compare the degree to which vaccine prevents diseases. Vaccine efficacy is the percentage reduction in cases of a disease in a vaccinated group compared to an unvaccinated group (or placebo). This is measured in controlled environments. The effectiveness of a vaccine is calculated after a vaccine is given to a larger population where real world setup is used to take the observational measurements. Figure 03 compares the different efficacy values of the existing COVID-19 vaccines (3).
Basic formula for Efficacy is calculated as below. (Orenstein, W A et al. “Field evaluation of vaccine efficacy.” Bulletin of the World Health Organization vol. 63,6 (1985): 1055-68.).
- Vaccine Efficacy =
Where, ARU= disease attack rate of unvaccinated people
ARV= disease attack rate of vaccinated people
Comparison between COVID-19 Vaccines
The chart below summarizes different COVID-19 vaccine technologies used globally (7,10).
| Name | Pfizer | Moderna | Johnson & Johnson | AstraZeneca | Sputnik V | Corona Vac | Sinopharm |
| Platform | BNT162b2 mRNA in lipid nanoparticle | mRNA-1273 mRNA in lipid nanoparticle | JNJ-78436725 Non-replicating human adenovirus/DNA | AZD 1222 Non-replicating Chimp Adenovirus-DNA | Ad26 and Ad5 adenovirus/DNA | BIBP-CorV and BBV152 | BIBP-CorV |
| Developer | Pfizer-BioNTech | Moderna | Johnson & Johnson | University of Oxford and AstraZeneca | Gamaleya Scientific research institute | Sinovac Biotech | Beijing Bio-Institute of Biological Products |
| Type
|
m-RNA | m-RNA | Viral Vector | Viral Vector | Viral Vector | Inactivated | Inactivated |
| Price (USD)
|
19.5 | 32-37 | 10 | 4 | ~10 | 14 | 30 |
| Storage Temperature
(Celsius) |
-80 to -60 | -25 to -15 | 2-8 | 2-8 | 2-8 | 2-8 | 2-8 |
| Efficacy | 95% | 94% | 72% | 69% | 92% | 50.4% | 79% |
| Number of Doses
|
2 | 2 | 1 | 2 | 2 | 2 | 2 |
| Protection from hospitalization | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| Adverse Effects | Anaphylaxis
(<0.001%) |
Anaphylaxis (<0.001%) | Anaphylaxis
(<0.001%), Thrombosis |
Thrombosis and Thrombocytopenia | None Reported | Fever
|
no serious side effects were reported |
| Time to immunity | 2 weeks after 2nd dose | 2 weeks after 2nd dose | Some protection at 2 weeks; maximal protection at 4 weeks | 15 days after 2nd dose | 2 weeks after 2nd dose | 15 days after 2nd dose | 2 weeks after the 2nd dose |
| Eligible Ages | >=16 | >=18 | >=18 | >=18 | 18 to 59 | 18 to 59 | 18 to 59 |
Limitations in Covid-19 vaccination
When it comes to vaccination campaigns, there are several challenges to face. Local health authorities as well as international health governing authorities are affected by it. There is a clear indication that vaccines are unfairly distributed around the world. To overcome that hurdle, the World Health Organization (WHO) has led the COVAX facility to maximize access to vaccines in every country. Also, some are hesitant to receive the vaccine due to their personal beliefs and misinformation [5] [9]. Therefore, local health authorities have the responsibility to educate people about the importance of receiving the vaccine [6].
Conclusion
As a global community, it is important to carry out vaccination programs with a central goal to prevent COVID-19 pandemic within a short time. We need to try our best to prevent the spread of the virus to stop mutations of the virus. As the general public it is our responsibility to get vaccinated timely and regularly. Otherwise, the effectiveness of the current vaccines will be reduced.
References
1. Worldometer. COVID-19 CORONAVIRUS PANDEMIC [Internet]. Worldometer. Available from: https://www.worldometers.info/coronavirus/ (accessed 15 December 2021).
2. Hampton T. Health agencies update. J Am Med Assoc. 2005;294(4):418. Available from: https://www.hamptonhealth.uk/news.aspx (accessed 14 December 2021).
3. van der Veldt AAM, Oosting SF, Dingemans AMC, Fehrmann RSN, GeurtsvanKessel C, Jalving M, et al. COVID-19 vaccination: the VOICE for patients with cancer.
4. Dodd RH, Pickles K, Nickel B, Cvejic E, Ayre J, Batcup C, et al. Concerns and motivations about COVID-19 vaccination. Lancet Infect Dis
5. Al-Qerem WA, Jarab AS. COVID-19 Vaccination Acceptance and Its Associated Factors Among a Middle Eastern Population[Internet]. Vol. 9, Frontiers in Public Health. 2021. p. 34.
- COVID-19 Vaccines | HIV, ID and Global Medicine. (n.d.). University of California San Francisco. Retrieved December 20, 2021.Available from: https://hividgm.ucsf.edu/covid-19-vaccines (accessed 25 December 2021).
- Rappuoli R, Pizza M, Del Giudice G, De Gregorio E. Vaccines, new opportunities for a new society. Proc Natl Acad Sci U S A. 2014;111(34):12288–93.
- Khubchandani J, Sharma S, Price JH, Wiblishauser MJ, Sharma M, Webb FJ. COVID-19 Vaccination Hesitancy in the United States: A Rapid National Assessment. J Community Health.
- Lu, C. (2021, April 27). How long does it take for the COVID-19 vaccines to become effective? Project PROTECH. Available from https://projectprotech.ca/front-page/how-long-does-it-take-for-the-covid-19-vaccines-to-become-effective/ (accessed 21 December 2021).


